Introduction
Ibrutinib was FDA approved for relapsed or refractory (R/R) marginal zone lymphoma (MZL) based on a phase II clinical trial that showed an overall response rate of 48% (Noy et al, Blood 2017). However, factors associated with response to ibrutinib in R/R MZL and outcomes of patients after progression on ibrutinib are unknown. Given the poor survival in other B-cell lymphomas such as mantle cell lymphoma (MCL) after progression on ibrutinib (Martin P et al, Blood 2016), we sought to evaluate clinicopathologic characteristics predictive of ibrutinib failure in R/R MZL, and describe outcomes of patients who experienced progression on ibrutinib therapy.
Methods
We performed a multicenter retrospective study of MZL patients treated at 19 US medical centers. Eligible patients were ≥ 18 years diagnosed with MZL from 2010-2019, who received ibrutinib for R/R MZL. Patients achieving a complete response (CR) or partial response (PR) with ibrutinib were considered ibrutinib responders, while those who had stable disease (SD) or progression of disease (PD) were classified as non-responders. The primary endpoint was to evaluate factors predictive of primary progression (PD) on ibrutinib. Secondary endpoints include evaluation of predictors of overall survival (OS) and progression-free survival (PFS) following ibrutinib therapy, assessment of outcomes based on the sequencing of ibrutinib therapy, and evaluation of outcomes following ibrutinib failure. PFS was defined as time from the start of ibrutinib therapy until lymphoma relapse/progression or death from any cause, censoring at last clinical assessment if no progression or death. OS was defined as time from the start of ibrutinib treatment until death or last follow‐up. A multivariable Poisson regression analysis was performed to model ibrutinib progression on the clinicopathologic factors (see Table). To identify significant predictors for OS and PFS, we used a multivariable Cox model.
Results
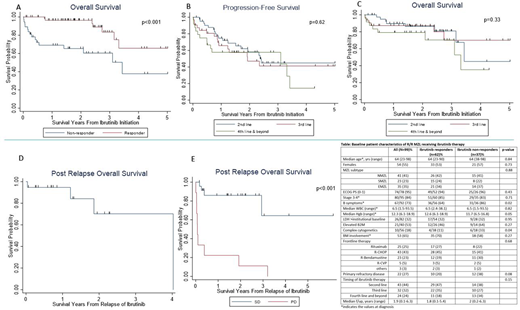
101 patients with R/R MZL received ibrutinib, of whom 99 had sufficient data for inclusion in the analysis. Among these patients, 63% (n=62) had CR/PR to ibrutinib (ibrutinib responders, CR=17, PR=45) and 37% (n=37) had no response (ibrutinib non-responders, SD=25, PD=12). The median duration of follow-up was 1.8 years (range=0.1-5.4 years) and 2 years (range=0.2-6.3 years) for ibrutinib responders and non-responders, respectively. Baseline characteristics of the R/R MZL patients stratified by ibrutinib response are shown in the Table. Among all the baseline factors examined for association with ibrutinib progression, only primary refractory disease (refractory to frontline therapy, RR=3.78, 95%CI=1.36-10.45, p=0.01) was predictive of a higher probability of primary progression on ibrutinib on multivariable analysis. The median OS was significantly better for responders (NR [not reached], 95%CI=3.2-NR) compared to non-responders (3.4 years, 95%CI=1.4-NR) (Figure 1A). Achieving CR/PR with ibrutinib (HR=0.22, 95%CI=0.09-0.52) and lack of complex cytogenetics (HR=0.22, 95%CI=0.08-0.59) were predictors of superior PFS. Similarly, ibrutinib response (HR=0.13, 95%CI=0.03-0.53) and lack of complex cytogenetics (HR=0.19, 95%CI=0.04-0.87) were predictors of better OS. There was no difference in PFS or OS based on the timing of ibrutinib administration (second vs third vs fourth line and beyond, Figure 1B and 1C). The median post ibrutinib relapse/progression OS (PROS) for patients who initially responded then progressed on ibrutinib (secondary progression, n=19) was 4 years (Figure 1D). The median PROS for patients who had no response to ibrutinib were stratified according to SD vs PD. The median PROS for those who had SD was NR and those with PD was 0.1 year (Figure 1E).
Conclusion
This is the largest series of R/R MZL patients treated with ibrutinib. A history of primary refractory disease was predictive of primary progression on ibrutinib, while the presence of complex cytogenetics was associated with inferior PFS and OS. In contrast to MCL, the outcomes of patients who progress on ibrutinib in R/R MZL are not poor except for the primary progression cohort (those with PD as the best response to ibrutinib). Improving therapeutic options for patients who experience PD with ibrutinib treatment represents an urgent unmet need and these patients should be prioritized for evaluation of novel therapeutic approaches.
Epperla:Verastem Oncology: Speakers Bureau; Pharmacyclics: Honoraria. Reddy:Genentech, BMS: Research Funding; KITE Pharma, Abbvie, BMS, Celgene: Consultancy. Caimi:Genentech: Research Funding; Celgene Corp: Other: Incyte Corporation - Ownership - Pharmacyclics, Inc. - Ownership - Celgene Corp. - Other, Speakers Bureau; ADC Therapeutics: Research Funding. Greenwell:Lymphoma Research Foundation: Research Funding; Acrotech Biopharma LLC, Kyowa Kirin: Consultancy. Janakiram:Takeda, Fate, Nektar: Research Funding. Olszewski:Genentech, Inc.: Research Funding; Spectrum Pharmaceuticals: Research Funding; TG Therapeutics: Research Funding; Adaptive Biotechnologies: Research Funding. Cohen:Genentech, BMS, Novartis, LAM, BioInvent, LRF, ASH, Astra Zeneca, Seattle Genetics: Research Funding; Janssen, Adicet, Astra Zeneca, Genentech, Aptitude Health, Cellectar, Kite/Gilead, Loxo: Consultancy. Palmisiano:AbbVie: Research Funding; Genentech: Research Funding. Awan:Genentech: Consultancy; Janssen: Consultancy; Astrazeneca: Consultancy; Abbvie: Consultancy; Pharmacyclics: Consultancy; Gilead Sciences: Consultancy; Kite Pharma: Consultancy; Dava Oncology: Consultancy; Celgene: Consultancy; Blueprint medicines: Consultancy; Sunesis: Consultancy; Karyopharm: Consultancy; MEI Pharma: Consultancy. Barta:Monsanto: Consultancy; Atara: Honoraria; Seattle Genetics: Honoraria, Research Funding; Janssen: Honoraria; Pfizer: Honoraria. Grover:Genentech: Research Funding; Tessa: Consultancy. Bartlett:Roche/Genentech: Consultancy, Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Affimed Therapeutics: Research Funding; Acerta: Consultancy; BTG: Consultancy; Seattle Genetics: Consultancy, Research Funding; Pharmacyclics: Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Millennium: Research Funding; Merck: Research Funding; Forty Seven: Research Funding; Immune Design: Research Funding; Janssen: Research Funding; Kite, a Gilead Company: Research Funding; ADC Therapeutics: Consultancy; Autolus: Research Funding; BMS/Celgene: Research Funding. Christian:Acerta: Research Funding; Celgene: Research Funding; Genentech: Research Funding; Merck: Research Funding; Millenium: Research Funding; MorphoSys: Research Funding; F Hoffman-La Roche: Research Funding; Triphase: Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Verastem: Membership on an entity's Board of Directors or advisory committees; AstraZenica: Membership on an entity's Board of Directors or advisory committees. Herrera:Pharmacyclics: Research Funding; Immune Design: Research Funding; AstraZeneca: Research Funding; Seattle Genetics: Consultancy, Research Funding; Gilead Sciences: Consultancy, Research Funding; Genentech, Inc./F. Hoffmann-La Roche Ltd: Consultancy, Research Funding; Merck: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Other: Travel, Accomodations, Expenses, Research Funding; Karyopharm: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal